近年来,基因疗法在治疗遗传病、罕见病等方面取得了重大进展,为患者带去新的治疗希望。截至今年3月9日,欧盟一共批准了10个基因疗法,详见下表。
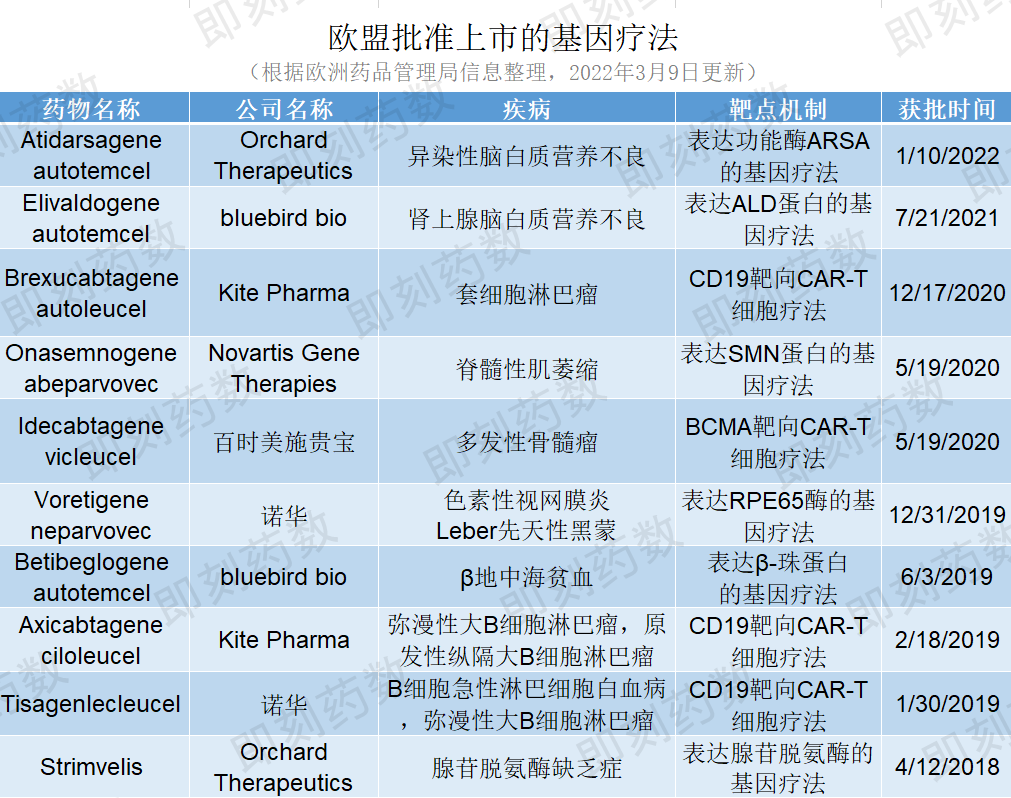
(药明康德内容团队制图,点击可见大图)
值得一提的是,今年有6个基因疗法可能在欧盟取得重大进展。其中,4个基因疗法有望获得欧盟批准,2个基因疗法提交上市授权申请(Marketing Authorisation Application,MAA),详见下表。
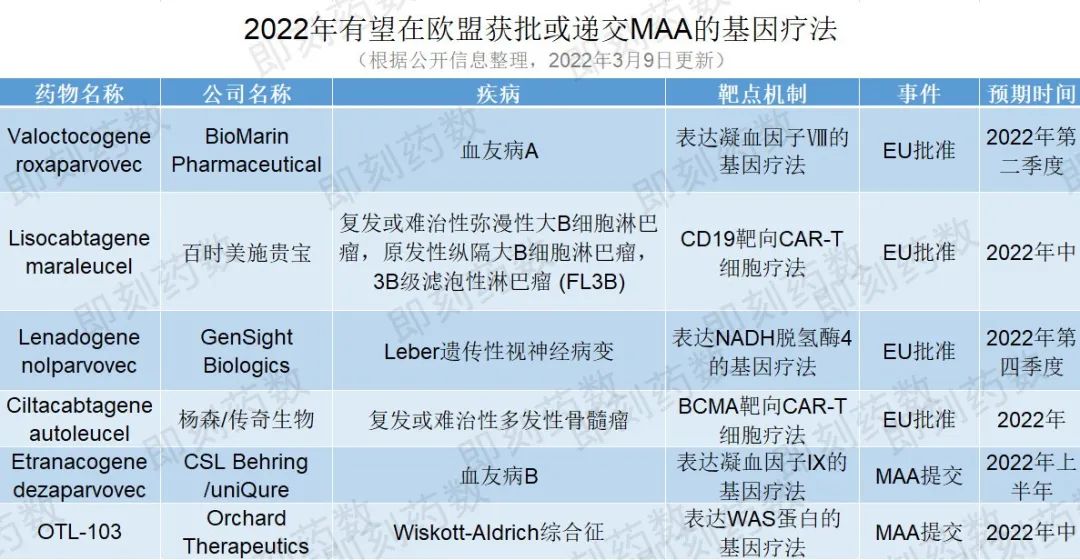
(药明康德内容团队制图,点击可见大图)
2022年在欧盟预期获批的基因疗法
第一个预期获批的基因疗法是BioMarin的valoctocogene roxaparvovec,一种基于AAV5载体的基因疗法,用于治疗严重的血友病A。该公司最近提交了积极的2年疗效数据以支持其MAA申报,预计人用药品委员会(Committee for Medicinal Products for Human Use,CHMP)意见将在2022年第二季度获得。如果获得批准,这可能是潜在的第一个治疗血友病A的基因疗法,根据公司发布信息,这款药物预计将于2022年第二季度获得批准。
另一个有希望获得批准的候选药物是百时美施贵宝的lisocabtagene maraleucel,一种以CD19为靶点的CAR-T细胞疗法,用于二线或多线全身治疗后的成人复发或难治性弥漫性大B细胞淋巴瘤(DLBCL)、原发性纵隔大B细胞淋巴瘤(PMBCL),滤泡性淋巴瘤3B级(FL3B)患者。CHMP在1月给出支持批准的积极意见,预计欧盟将在2022年年中做出批准决定。该疗法已获美国FDA批准。
同样值得关注的是GenSight Biologics的lenadogene nolparvovec,一种编码人类野生型ND4蛋白的AAV2基因疗法,用于治疗Leber遗传性视神经病变,这是一种罕见的线粒体疾病,在青少年时就会发病,导致不可逆转的失明。欧盟目前正在对提交的MAA进行监管审评。GenSight预计该申请将在2022年第四季度获得批准,并计划在2023年第一季度推出此治疗方式。
最后一个潜在的欧盟批准候选药物是杨森和传奇生物的ciltacabtagene autoleucel,一种靶向B细胞成熟抗原(BCMA)的自体CAR-T细胞免疫疗法,用于治疗复发或难治性多发性骨髓瘤。目前公司正在等待对其MAA的监管决定。这种疗法已经成为杨森在美国获批的首个细胞疗法。如果在欧盟获得批准,该疗法可能成为欧盟第二个针对多发性骨髓瘤的BCMA靶向细胞疗法。
2022年在欧盟预期提交MAA的基因疗法
除了潜在的批准外,预计今年还会有两种基因疗法会进行MAA的提交。uniQure和CSL Behring的etranacogene dezaparvovec是一种基于AAV5载体的基因疗法,处于3期临床试验中,用于治疗血友病B,这是一种危及生命的出血性疾病。CHMP已准许使用加速评估程序对这一MAA进行审评,计划于2022年上半年进行。一旦MAA提交完成并被欧盟监管机构接受,加速评估可能会将审查时间从210天缩短到150天。
Orchard Therapeutics的OTL-103是一种离体、自体、造血干细胞(HSC)基因疗法,用于治疗Wiskott-Aldrich综合征,这是一种罕见的遗传性免疫缺陷疾病,目前已知的治疗方法是干细胞移植。在与EMA进行有效的监管互动后,该公司计划在2022年年中提交MAA。
2022 EU Regulatory Outlook for Gene Therapy
After fifty years of ebb and flow, gene therapy has made significant progress in the treatment of genetic diseases and rare diseases. The number of approved gene therapies in the EU was ten by March 9, 2022.
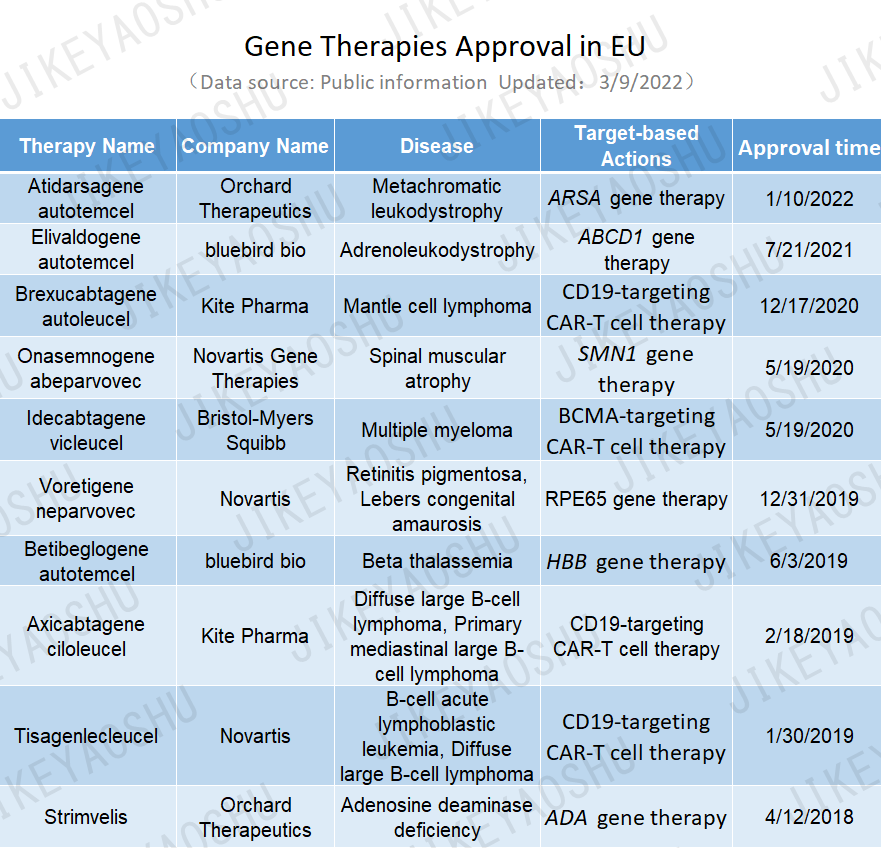
(By WuXi AppTec content team. Click the image to view at full size)
Will gene therapy continue to set sail in the EU this year? We could witness the potential approval of four products in the EU and two MAA submission by the end of this year.
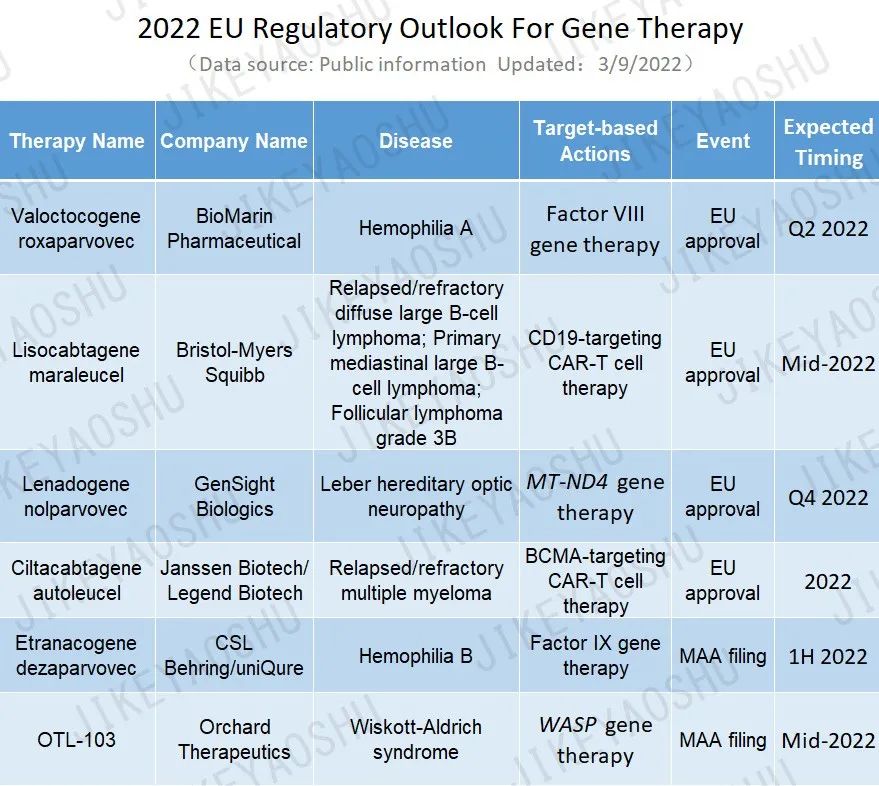
(By WuXi AppTec content team. Click the image to view at full size)
Gene therapies expected to be approved in the EU in 2022
These include BioMarin’s valoctocogene roxaparvovec, an AAV5 gene therapy, for the treatment of severe hemophilia A. The company has recently submitted positive 2-year efficacy data to support its MAA filing and a CHMP opinion is expected for the Q2 2022. If approved, this could potentially be the first gene therapy for hemophilia A, with an anticipated launch in 2H 2022.
Another promising candidate is Bristol Myers Squibb’s lisocabtagene maraleucel, a CD19-directed CAR-T cell therapy, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma grade 3B (FL3B) after two or more lines of systemic therapy. Following CHMP’s recommendation in January 2022, it is anticipating EU approval decisions around mid-2022. The therapy has been approved in the US for these settings.
Also worth our attention is GenSight Biologics’ lenadogene nolparvovec, an AAV2 gene therapy encoding the human wild-type ND4 protein, for the treatment of Leber hereditary optic neuropathy, a rare mitochondrial disease that leads to irreversible blindness. EU regulatory review for the submitted MAA is ongoing at this time. GenSight is preparing for potential approval in Q4 2022 and launch in Q1 2023.
The last potential EU approval candidate is Janssen & Legend Biotech’s ciltacabtagene autoleucel, a B-cell maturation antigen (BCMA)-targeting genetically modified autologous T cell immunotherapy for the treatment of relapsed or refractory multiple myeloma, awaiting regulatory decisions for its MAA in 2022. Last month, it became Janssen’s first cell therapy approved in the US. If approved in the EU, the therapy could become the second BCMA-targeting cell therapy for multiple myeloma in the EU.
Expected Gene Therapy MAA submission in 2022
Besides the potential approvals, we are expecting to see two MAA submissions this year. uniQure and CSL Behring’s etranacogene dezaparvovec is an AAV5-based gene therapy in phase III trials for the treatment of hemophilia B, a life-threatening bleeding disorder. The CHMP has granted the therapy accelerated assessment for its MAA, which is planned for 1H2022. The accelerated assessment potentially reduces the review timeline from 210 days to 150 days once the MAA is filed and validated.
Orchard Therapeutics’ OTL-103 is an ex vivo, autologous, hematopoietic stem cell (HSC) gene therapy in registration trials for the treatment of Wiskott-Aldrich syndrome, a rare inherited immune deficiency disorder which the known cure so far is a stem cell transplant. Following productive regulatory interactions with the EMA, the company is planning to file an MAA in mid-2022.
参考资料:
[1] BioMarin Announces Fourth Quarterand Full Year 2021 Financial Results and Corporate Updates. Retrieved March 8, 2022 from, https://investors.biomarin.com/2022-02-23-BioMarin-Announces-Fourth-Quarter-and-Full-Year-2021-Financial-Results-and-Corporate-Updates
[2] BioMarin Provides Updates onProgress in Gene Therapy Programs. Retrieved March 8, 2022 from, https://investors.biomarin.com/2022-02-17-BioMarin-Provides-Updates-on-Progress-in-Gene-Therapy-Programs
[3] Bristol Myers Squibb ReceivesPositive CHMP Opinion for CAR T Cell Therapy Breyanzi (lisocabtagenemaraleucel) for Relapsed or Refractory DLBCL, PMBCL and FL3B. Retrieved March 8, 2022 from, https://news.bms.com/news/corporate-financial/2022/Bristol-Myers-Squibb-Receives-Positive-CHMP-Opinion-for-CAR-T-Cell-Therapy-Breyanzi-lisocabtagenemaraleucel-for-Relapsed-or-Refractory-DLBCL-PMBCL-and-FL3B/default.aspx
[4] Corporate Presentation of GenSight Biologics. Retrieved March 8, 2022 from, https://www.gensight-biologics.com/wp-content/uploads/2022/02/GenSight-Biologics-Corporate-presentation-February-2022-v25022022.pdf
[5] U.S. FDA Approves CARVYKTI™(ciltacabtagene autoleucel), Janssen’s First Cell Therapy, a BCMA-DirectedCAR-T Immunotherapy for the Treatment of Patients with Relapsed or RefractoryMultiple Myeloma. Retrieved March 8, 2022 from, https://www.janssen.com/us-fda-approves-carvykti-ciltacabtagene-autoleucel-janssens-first-cell-therapy-bcma-directed-car-t
[6] GenScript Biotech Reports FirstHalf 2021 Results Posts explosive growth in GCT CDMO business and strategic GCTinvestments. Retrieved March 8, 2022 from, https://www.genscript.com/genscript-biotech-2021-interim-results-presentation.html?page_no=1&position_no=2&sensors=search
[7] First cell-based gene therapy totreat adult patients with multiple myeloma. Retrieved March 8, 2022 from, https://www.ema.europa.eu/en/news/first-cell-based-gene-therapy-treat-adult-patients-multiple-myeloma
[8] CSL Behring Receives AcceleratedCHMP Assessment for Etranacogene Dezaparvovec for European Patients Living withHemophilia B. Retrieved March 8, 2022 from, https://www.cslbehring.com/newsroom/2021/chmp-accelerates-etranadez-application
[9] uniQure and CSL Behring AnnouncePrimary Endpoint Achieved in HOPE-B Pivotal Trial of Etranacogene DezaparvovecGene Therapy in Patients with Hemophilia B. Retrieved March 8, 2022 from, https://www.cslbehring.com/newsroom/2021/hope-b-gene-therapy-for-hemophilia-b-topline-results
[10] Orchard Therapeutics AnnouncesRecent Commercial and Regulatory Progress for Late-stage HSC Gene TherapyPrograms and Outlines Key 2022 Milestones. Retrieved March 8, 2022 from, https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-recent-commercial-and-regulatory
[11] Pipeline of Orchard Therapeutics.Retrieved March 8, 2022 from, https://www.orchard-tx.com/approach/pipeline/
[12] Wiskott-Aldrich Syndrome. Retrieved March 8, 2022 from, https://www.childrenshospital.org/conditions-and-treatments/conditions/w/wiskott-aldrich-syndrome
关于举办2026年度四川省药品生产企业质
各药品生产企业: 2026年是我国..四川省医药保化品质量管理协会召开第七
2025年12月17日,四川省医药保化品..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..四川省医药保化品质量管理协会党支部开
为庆祝中国共产党成立104周年,持..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..关于相关收费标准的公示
根据四川省医药保化品质量管理协会..关于收取2025年度会费的通知
各会员单位: 在过去的一年里,..“两新联万家,党建助振兴”甘孜行活动
为深入贯彻落实省委两新工委、省市..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..