去年,美国FDA批准了50种新药。2022年第一季度,FDA已经继续批准了7种新药,并预计在本月底之前再做出5项审评决定,详见下表。
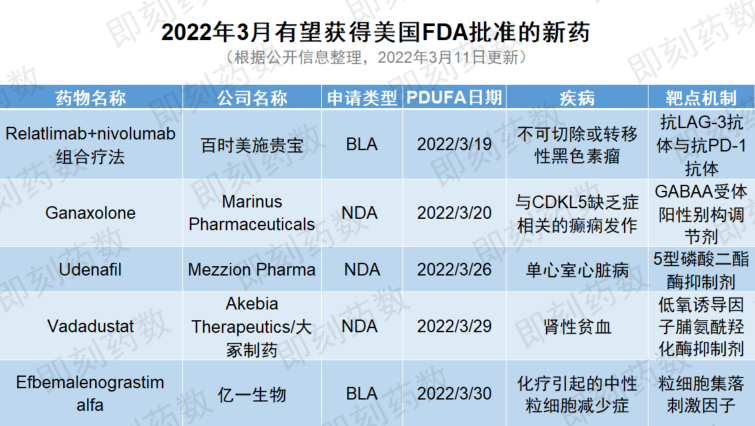
1. 新药名称:relatlimab+nivolumab固定剂量组合疗法(FDC)
研发公司:百时美施贵宝
治疗疾病:不可切除或转移性黑色素瘤
目前PDUFA日期排在第一位的是百时美施贵宝的relatlimab+nivolumab固定剂量组合疗法(FDC),用于治疗不可切除或转移性黑色素瘤。这一生物制品许可申请(BLA)得到了积极的3期安全性和有效性数据的支持,这些数据显示这一组合治疗方式在转移性黑色素瘤中相对于抗PD-1抗体单一疗法具有统计学意义和临床意义的无进展生存益处。FDA预计将在2022年3月19日之前做出决定。经批准后,这可能是抗LAG-3单克隆抗体与抗PD-1单克隆抗体的潜在首个FDC疗法。
2. 新药名称:加奈索酮(ganaxolone)
研发公司:Marinus Pharmaceuticals
治疗疾病:与CDKL5缺乏症相关的癫痫发作
Marinus Pharmaceuticals公司的加奈索酮(ganaxolone)口服混悬剂是一种γ-氨基丁酸(GABAA)受体别构调节剂。2021年7月Marinus公司在美国提交了新药申请(NDA),用于治疗与细胞周期蛋白依赖性激酶样5(CDKL5)缺乏症相关的癫痫发作,这是一种罕见的遗传性癫痫,迄今尚未有治疗方式获批。2021年9月,Marinus公司宣布,美国FDA已经授予ganaxolone的NDA优先审评资格,预计其PDUFA日期为今年3月20日。该药物预计将于2022年6月上市,具有潜力满足多种罕见癫痫未满足的需求。
3. 新药名称:udenafil
研发公司:Mezzion Pharma
治疗疾病:单心室心脏病
Mezzion Pharma公司的udenafil是一种长效、选择性、口服5型磷酸二酯酶(PDE5)抑制剂,用于提高12岁及以上患有单心室心脏病(SVHD)、已接受了方谭氏(Fontan)手术的患者的运动能力。虽然方谭氏手术创造了稳定的循环,患者单心室可将血液泵入身体,但由于运动能力下降,在方谭氏手术方完成后的二三十年内,患者住院和心源性死亡的风险增加。2022年2月Mezzion与FDA举行了后期审查会议(Late-Cycle review Meeting),在会上解释了提交的数据。如果在2022年3月26日或之前获得FDA批准,udenafil将是潜在的第一个获批治疗这种罕见儿科疾病人群的疗法。
4. 新药名称:vadadustat
研发公司:Akebia Therapeutics/大冢制药
治疗疾病:肾性贫血
Akebia公司与大冢制药的vadadustat,是一种潜在的“first-in-class”缺氧诱导因子脯氨酰羟化酶(HIF-PH)抑制剂,用于口服治疗慢性肾病(CKD)引起的贫血。这种药物已经在日本上市销售。3期临床结果表明,透析依赖人群达到了安全性和有效性终点。该药物的PDUFA日期为今年3月29日。
5. 新药名称:efbemalenograstim alfa
研发公司:亿一生物
治疗疾病:化疗引起的中性粒细胞减少症
最后,亿一生物的efbemalenograstim alfa是一种二聚体重组人粒细胞集落刺激因子(rhG-CSF)与人IgG2-Fc片段构成的融合蛋白,预计FDA将于2022年3月30日做出决定。基于两项全球3期临床试验的安全性和有效性数据,该公司于2021年3月提交了BLA,用于化疗引起的中性粒细胞减少症。该药物在欧盟的新药上市申请(MAA)也在审查当中。上个月,中国国家药监局(NMPA)受理了该药在中国的上市申请。由于其独特的结构,如果获得批准,它将提供一种长效治疗选择,以替代目前的聚乙二醇化G-CSF治疗方式。
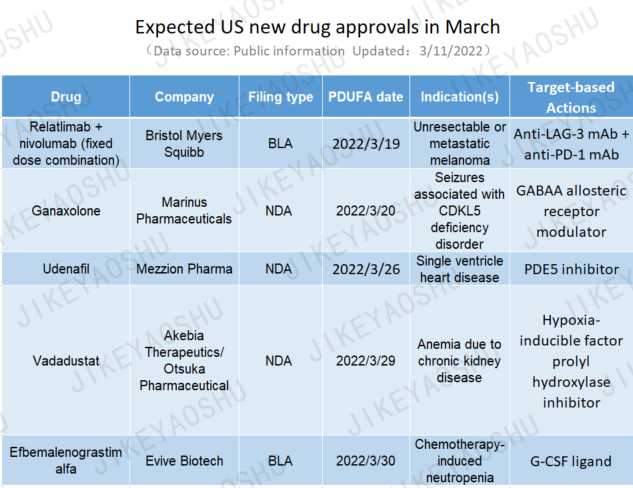
Expected US New Drug Approvals in March
Last year, the US FDA approved 50 new drugs. For the first quarter of 2022, it has so far approved seven and is expecting to make decisions on five more by the end of this month.
(By WuXi AppTec content team. Click the image to view at full size)
1. Drug: Relatlimab + nivolumab (fixed dose combination)
Company: Bristol Myers Squibb
Indication(s): Unresectable or metastatic melanoma
The first FDA decision to be made in March is on Bristol Myers Squibb’s relatlimab plus nivolumab fixed-dose combination (FDC), seeking marketing approval for the treatment of unresectable or metastatic melanoma. The BLA filing was supported by positive phase III safety and efficacy data which showed statistically significant and clinically meaningful progression-free survival benefit of the FDC over anti-PD-1 monotherapy in metastatic melanoma. The FDA is expected to make a decision by March 19, 2022. Upon approval, this could be the first-in-class FDC therapy of anti-LAG3 mAb plus anti-PD-1 mAb.
2. Drug: ganaxolone
Company: Marinus Pharmaceuticals
Indication(s): Seizures associated with CDKL5 deficiency disorder
This is closely followed by Marinus’ oral suspension formulation of ganaxolone, a positive allosteric GABAA receptor modulator. An NDA was submitted in the US in July 2021 for the treatment of seizures associated with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder, a rare, genetic epilepsy with no disease-specific treatments approved hitherto. The company expects PDUFA action date to be on March 20, 2022 for the oral formulation, with anticipated launch in June 2022. The drug has potential for expansion to complimentary clinical indications to address unmet needs in multiple rare epilepsies.
3. Drug: udenafil
Company: Mezzion Pharma
Indication(s): Single ventricle heart disease
Mezzion’s udenafil is a long-acting, selective, oral phosphodiesterase-5 (PDE5) inhibitor, awaiting US approval as a therapeutic to improve the exercise capacity of patients 12 years of age and older with single ventricle heart disease (SVHD) who have undergone the Fontan operation. While the Fontan procedure creates a stable circulation to allow the single ventricle to pump blood to the body, the risk of hospitalization and cardiac death increases in the second and third decades after Fontan completion, due to a decline in exercise capacity. A late cycle review meeting was held with the FDA in February 2022 to explain the data submitted. If approved by the FDA on or before the PDUFA date of March 26, 2022, udenafil would potentially be the first approved therapy for this rare pediatric disease population.
4. Drug: vadadustat
Company: Akebia Therapeutics/Otsuka Pharmaceutical
Indication(s): Anemia due to chronic kidney disease
The next in line to be reviewed by the FDA is Akebia’s vadadustat, a potential first-in-class hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor for the oral treatment of anemia due to chronic kidney disease (CKD). The drug is already marketed in Japan for this setting. Phase III results indicated both safety and efficacy endpoints were met from the dialysis-dependent population. The PDUFA date of vadadustat is March 29, 2022.
5. Drug: efbemalenograstim alfa
Company: Evive Biotech
Indication(s): Chemotherapy-induced neutropenia
Finally, Evive’s efbemalenograstim alfa, a dimeric recombinant human granulocyte colony stimulating factor (rhG-CSF)-fc fusion protein, is expecting FDA approval decision on March 30, 2022. The company submitted a BLA in March 2021 for the drug for chemotherapy-induced neutropenia, based on the positive phase III safety and efficacy data from two global trials. An MAA is also under review in the EU. Last month, the NMPA accepted marketing application of the drug in China. Owing to its unique structure, it would present a long-acting alternative to the current standard of care pegylated G-CSF treatments.
参考资料:
[1] New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products. Retrieved March 11, 2022 from, https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products
[2] Corporate Presentationof Marinus. Retrieved March 11, 2022 from, https://s21.q4cdn.com/104148044/files/doc_presentations/2022/Bristol-Myers-Squibb-JPM-2022-Presentation.pdf
[3] Marinus PharmaceuticalsSubmits New Drug Application (NDA) to FDA for Ganaxolone for the Treatment of Seizures Associated with CDKL5 Deficiency Disorder and Provides Pipeline Update. Retrieved March 11, 2022 from, https://ir.marinuspharma.com/news/news-details/2021/Marinus-Pharmaceuticals-Submits-New-Drug-Application-NDA-to-FDA-for-Ganaxolone-for-the-Treatment-of-Seizures-Associated-with-CDKL5-Deficiency-Disorder-and-Provides-Pipeline-Update/default.aspx
[4] Corporate Presentationof Marinus. Retrieved March 11, 2022 from, https://s25.q4cdn.com/443656056/files/doc_downloads/2021/12/Corporate-Deck-December-FINAL.pdf
[5] Mezzion's New DrugApplication ("NDA") for its Orphan Drug Udenafil for the Treatment of Single Ventricle Heart Disease ("SVHD") has been Accepted for Filingby the FDA. Retrieved March 11, 2022 from, https://www.prnewswire.com/news-releases/mezzions-new-drug-application-nda-for-its-orphan-drug-udenafil-for-the-treatment-of-single-ventricle-heart-disease-svhd-has-been-accepted-for-filing-by-the-fda-301299410.html
[6] Late-Cycle review Meeting Notice. Retrieved March 11, 2022 from, https://www.mezzion.co.kr/bbs/board.php?bo_table=notice&wr_id=106
[7] Akebia Therapeutics Reports Fourth Quarter and Full-Year 2021 Financial Results and Recent Business Highlights. Retrieved March 11, 2022 from, https://ir.akebia.com/news-releases/news-release-details/akebia-therapeutics-reports-fourth-quarter-and-full-year-2021
[8] BETTERING THE LIVES OF PEOPLEIMPACTED BY KIDNEY DISEASE. Retrieved March 11, 2022 from, https://ir.akebia.com/static-files/7bb8acb3-1543-4ce5-9154-37ccb4157671
[9] Update on US regulatory review of roxadustat in anaemia of chronic kidney disease. Retrieved March 11, 2022 from, https://www.astrazeneca.com/media-centre/press-releases/2021/update-on-us-review-of-roxadustat.html
[10] Evive Biotech Submits Biologics License Application to US FDA for Ryzneuta(TM). Retrieved March 11, 2022 from, https://www.prnewswire.com/news-releases/evive-biotech-submits-biologics-license-application-to-us-fda-for-ryzneutatm-301259250.html
[11]欧洲药品管理局正式受理亿一生物Ryzneuta™的上市许可申请。RetrievedMarch 11, 2022 from, https://www.yifanyy.com/qiyeyaowen/detail/2094.html
[12] F-627上市申请获得国家药监局正式受理。Retrieved March 11, 2022 from, https://www.yifanyy.com/qiyeyaowen/detail/2125.html
关于举办2026年度四川省药品生产企业质
各药品生产企业: 2026年是我国..四川省医药保化品质量管理协会召开第七
2025年12月17日,四川省医药保化品..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..协会党支部组织党日主题学习会
协会党支部组织党日主题学习会 --..四川省医药保化品质量管理协会党支部开
为庆祝中国共产党成立104周年,持..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..关于相关收费标准的公示
根据四川省医药保化品质量管理协会..关于收取2025年度会费的通知
各会员单位: 在过去的一年里,..“两新联万家,党建助振兴”甘孜行活动
为深入贯彻落实省委两新工委、省市..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..