近年来,靶向蛋白降解是新药研发的热点领域,可以用于靶向此前不可成药的靶点,通过降解与疾病相关的蛋白治疗多种疾病,解决传统小分子或生物大分子无法解决的难题。
小分子靶向蛋白降解药物(TPDs)诸如蛋白降解靶向嵌合体(PROTACs)、CRBN E3连接酶调节剂(CELMoDs)、分子胶等,被设计用于靶向疾病相关的细胞内蛋白,通过将其传输到泛素-蛋白酶体系统进行降解。这些靶向蛋白降解药物具有许多优点,包括其催化机制、细胞内渗透性和靶向多种蛋白的潜力。
自Arvinas于2019年将首个PROTAC推入临床试验以来,根据公开信息不完全统计,截至2022年3月9日,全球共有临床阶段蛋白降解药物24个,其中中国公司开发的临床阶段药物有5个。靶向蛋白降解药物领域正在进入一个发展的重要阶段。首批PROTACs的早期临床试验成功证明了基本的安全性和有效性,它们发展的下一个阶段是完成3期临床试验和注册。业界正在积极地追踪这些研究,希望看到审批的进展。
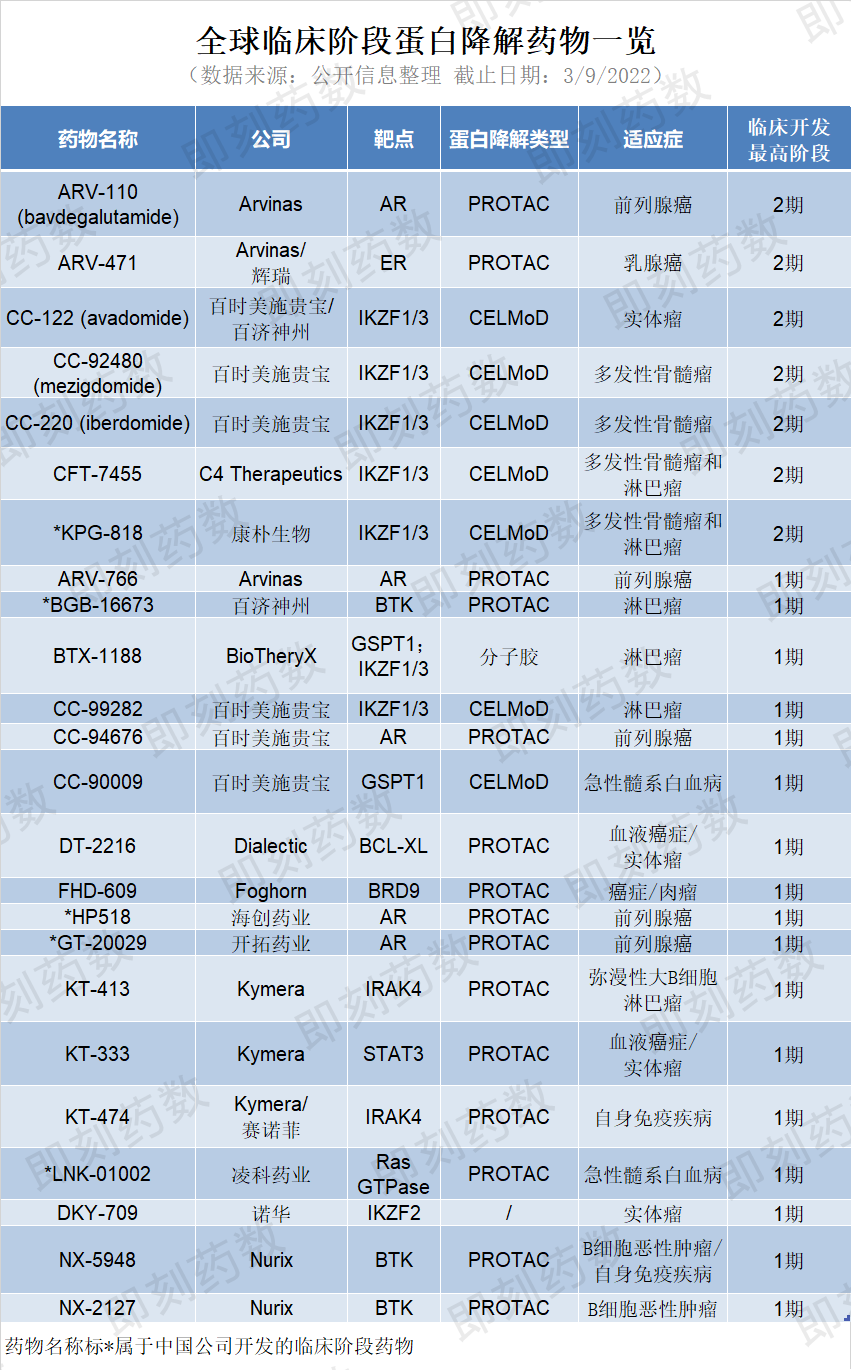
(药明康德内容团队制图,点击可见大图)
开发靶向蛋白降解药物的主要公司预计,2022年会有重要的里程碑。下面将精选7家2022年可能有重大研发进展的公司进行盘点。
Arvinas
Arvinas于2019年将首批PROTAC药物ARV-110(bavdegalutamide)和ARV-471(与辉瑞合作)引入临床。2022年,Arvinas预计将公布ARV-110单药治疗去势抵抗性前列腺癌的最终2期ARDENT数据,以及ARV-110与abiraterone联合治疗的1b期数据。由于新冠疫情,一项3期临床试验的入组于2020年暂停。对于ARV-471,今年还将公布VERITAC单药治疗ER+/HER2-乳腺癌剂量扩展临床试验的2期数据。该公司第三种临床药物ARV-766有望在年底前完成1期剂量递增研究。ARV-766与ARV-110靶向的同样是雄激素受体,用于前列腺癌的治疗。关于这一疗法,Arvinas并未透露太多,只说它与ARV-110具有差异化的特性。
百时美施贵宝(BMS)
百时美施贵宝(BMS)开发的靶向蛋白降解药物iberdomide和mezigdomide都处于2期开发阶段,用于多发性骨髓瘤(MM)。BMS将iberdomide视为“早期MM治疗的新支柱”。2022年,该公司打算开始iberdomide的3期EXCALIBER临床试验。他们还计划在下半年公布mezigdomide治疗多发性骨髓瘤的1/2期临床试验结果。在2021年11月的投资者活动中,BMS报告称,至少还有11种蛋白降解药物处于临床前及1期临床试验的早期开发阶段。
Kymera Therapeutics
Kymera目前有3个临床阶段的PROTAC药物。KT-474是一种靶向IRAK4治疗免疫炎症的药物,目前正与赛诺菲合作进行1期临床试验。Kymera希望在今年下半年报告试验进展。Kymera还打算在今年晚些时候报告另外两个PROTAC药物的临床试验进展。
Nurix Therapeutics
Nurix Therapeutics于2021年已经将4个项目推向临床,公司预计将进一步推进这些项目,并在2022年公布初步结果。其中两个项目是PROTAC药物,NX-2127和NX-5948;均针对B细胞恶性肿瘤的布鲁顿酪氨酸激酶(BTK)。2022年,公司计划在年中启动NX-2127 1a/1b期研究的1b期扩展,用于复发/难治性B细胞恶性肿瘤患者,并于2022年下半年报告1a期结果。对于NX-5948,Nurix Therapeutics计划在英国开始1期给药,并在年底前报告安全性和PK数据。
C4 Therapeutics
C4 Therapeutics有1个药物CFT-7455处于多发性骨髓瘤和非霍奇金淋巴瘤的2期临床开发阶段。2022年4月,公司将在AACR会议上报告临床前和1/2期临床数据。
Foghorn Therapeutics
Foghorn Therapeutics目前正在研究至少8种靶向蛋白降解药物。进展最快的药物FHD-609靶向BRD9的癌症适应症,正处于滑膜肉瘤的1期研究。预计在2022年上半年报告1期研究的早期结果。
海创药业(Hinova Pharmaceuticals)
2022年1月,海创药业(Hinova Pharmaceuticals)开展一种高度选择性和口服生物可利用的靶向雄激素受体(AR)的蛋白降解药物HP518的1期临床试验,首个转移性去势抵抗性前列腺癌(mCRPC)患者已成功入组。正在澳大利亚进行的1期开放标签研究将评估HP518在mCRPC患者中的安全性、药代动力学和抗肿瘤活性。
Drug developers continue to seek new ways to attack difficult protein targets and expand the druggable proteome. The challenge is to find new modalities that engage these difficult targets where traditional small molecules or larger biomolecules have failed. A flexible approach to targeted protein degradation that engages a multiplicity of disease-causing protein targets and eliminates them from within or outside of a cell could be broadly applicable means of addressing traditionally undruggable targets.
Modalities such as proteolysis targeting chimeras (PROTACs), cereblon E3 ligase modulators (CELMoDs), molecular glues and a host of similar approaches are being designed to target and eliminate disease-associated intracellular proteins by shuttling them to the ubiquitin-proteasome system for degradation. These small molecules, targeted protein degraders (TPDs) have a number of advantages including their catalytic mechanism, intracellular permeability and potential to target a wide variety of proteins.
The first PROTACs were taken into the clinic by Arvinas in 2019. While these drugs have been progressing through clinical testing, others have entered trials and even more are in pre-clinical development and early discovery. According to incomplete statistics of public information, there were 24 clinical stage protein degradation drugs in the world, among which 5 were developed by Chinese companies by 3/9/2022. The TPD field is approaching an important phase of its evolution as new drugs enter the clinic and the first wave reaches mid-to-late-stage clinical checkpoints. Early successes of the first PROTACs have demonstrated basic safety and efficacy. The next phase in their evolution is to reach and complete phase 3 and registrational trials. The industry is anxiously tracking these studies, hoping to see progression toward approvals.
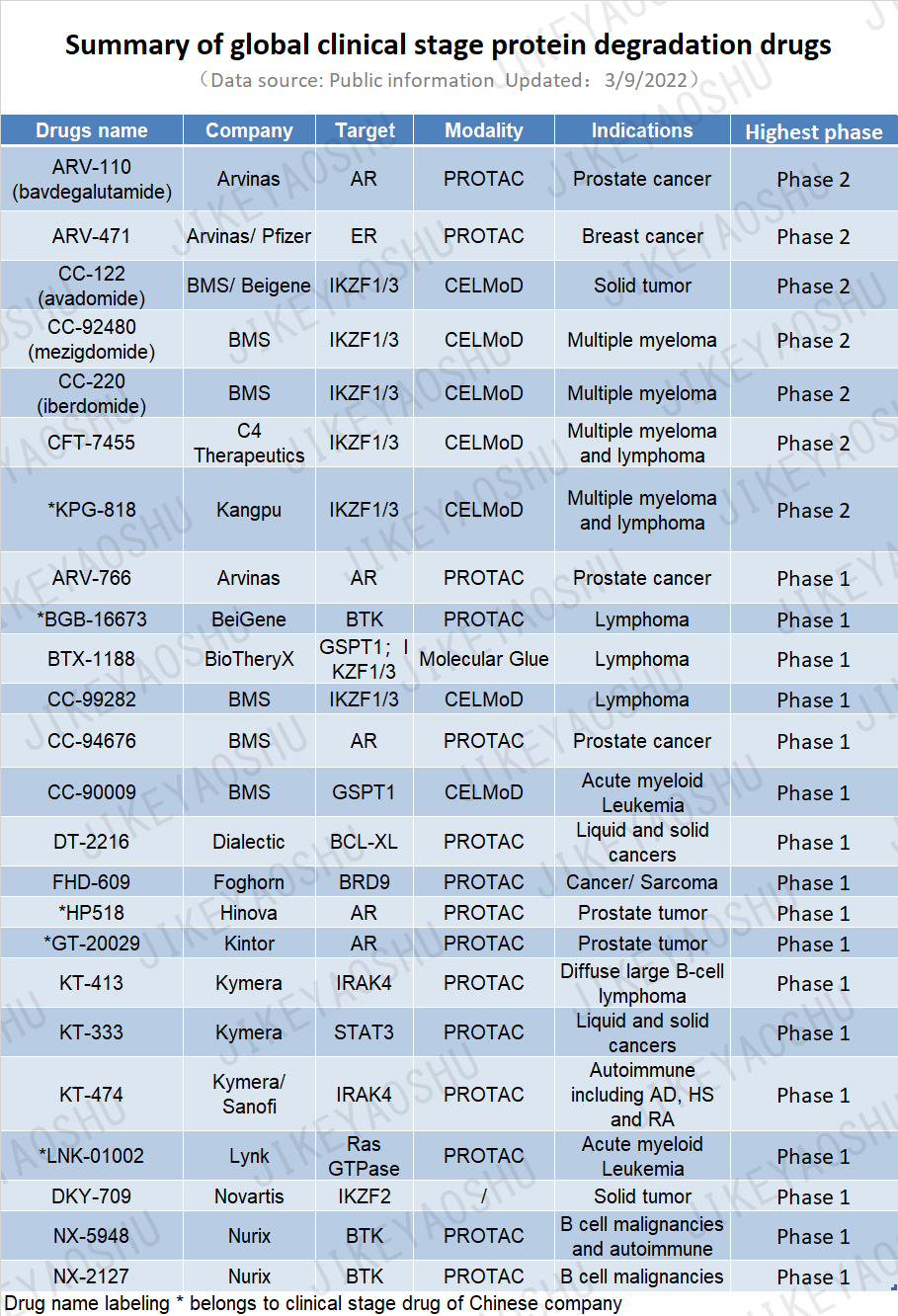
(By WuXi AppTec content team. Click the image to view at full size)
Leading developers anticipate important milestones for their drugs in 2022. There are seven companies that could make major R&D advances in 2022.
Arvinas
Arvinas leads the pack with the first PROTACs, bavdegalutamide (ARV-110) and ARV-471 (partnered with Pfizer), in the clinic since 2019. In 2022 Arvinas expects to report final phase 2 ARDENT data for ARV-110 mono therapy for castration resistant prostate cancer and phase 1b data for its combination with abiraterone. Enrollment for a phase 3 trial was paused in 2020 due to COVID-19. For ARV-471, phase 2 data from the VERITAC mono therapy dose expansion trials in ER+/HER2- breast cancer will also be reported this year. The company’s third clinical drug, ARV-766 is expected to complete a phase 1 dose escalation study by the end of the year. ARV-766 targets the same androgen receptor as ARV-110, which is used in the treatment of prostate cancer. Arvinas isn't saying much about the treatment, other than that it has characteristics that differentiateit from ARV-110.
Bristol Myers Squibb(BMS)
BMS is developing 2 CELMoDs. Iberdomide and mezigdomide are in phase 2 development for multiple myeloma (MM). BMS sees iberdomide as “a new backbone in early line MM” treatment. In 2022, the company intends to start its phase 3 EXCALIBER trial for iberdomide. They also plan on an important read-out of phase1/2 trial results for mezigdomide in multiple myeloma in the second half of the year. In a November 2021 investor event, BMS reported at least 11 more TPDs in its early stage pipeline.
Kymera Therapeutics
Kymera currently has 3 clinical-stage PROTACs. KT-474, targeting IRAK4 for immune-inflammatory conditions is in phase 1 trials in partnership with Sanofi. Kymera expects to report proof of benefit results in the second half of the year. Kymera also intends to report proof-of-benefit for it’s two other PROTACs later in the year.
Nurix Therapeutics
In 2021, Nurix pushed 4 programs into the clinic and the company is expecting to advance these and report early results in 2022. Two of these programs are PROTACs, NX-2127 and NX-5948; both targeting Bruton’s Tyrosine Kinase for B-cell malignancies. In 2022, the company plans to initiate phase 1b expansion of the NX-2127 phase 1a/1b study in patients with relapsed/refractory B-cell malignancies mid-year and report phase 1a results in the second half of 2022. For NX-5948, Nurix plans to begin phase 1 dosing in the UK and report safety and PK data toward the end of the year.
C4 Therapeutics
C4 Therapeutics has 1 CELMoD (CFT-7455) in phase 2 clinical development for multiple myeloma and non-Hodgkin’s lymphoma. In April of 2022, the company will be reporting preclinical and phase 1/2 clinical data at AACR.
Foghorn Therapeutics
Foghorn Therapeutics is currently pursuing >8 targeted protein degraders. FHD-609 targets BRD9 for cancer indications and is in phase 1 for synovial sarcoma. Foghorn anticipates reporting the early results of this phase 1 study in the first half of 2022.
Hinova Pharmaceuticals
The first patient with metastatic castration-resistant prostate cancer (mCRPC) has been successfully dosed in a phase 1 clinical trial of HP518, a highly selective and orally bioavailable chimeric degrader targeting androgen receptor (AR) in 2022. The ongoing open-label phase 1 study in Australia will evaluate the safety, pharmacokinetics, and anti-tumor activity of HP518 in patients with mCRPC.
四川省医药保化品质量管理协会党支部开
为庆祝中国共产党成立104周年,持..四川省医药保化品质量管理协会党支部召
四川省医药保化品质量管理协会党支..认真落实巡视组反馈意见,进一步规范协
按照四川省市场监督管理局党组巡视..关于相关收费标准的公示
根据四川省医药保化品质量管理协会..关于召开会长办公会的通知
各会长、副会长单位: 根据四川..四川省医药保化品质量管理协会组织召开
2025版《中国药典》将于2025年10月..四川省医药保化品质量管理协会召开第七
四川省医药保化品质量管理协会第七..“两新联万家,党建助振兴”甘孜行活动
为深入贯彻落实省委两新工委、省市..关于收取2025年度会费的通知
各会员单位: 在过去的一年里,..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..四川省应对新型冠状病毒肺炎疫情应急指
四川省应对新型冠状病毒肺炎疫情应..